
Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
BAPEN / BIFA Guidelines on the Diagnosis and Management
of Intestinal Failure Associated Liver Disease (IFALD)
Authors**: Dr Lisa Sharkey and the BIFA committee
** Competing interests: None declared
August 2022
Aims of the Guidelines
1. To review the aetiopathogenesis and risk factors of IFALD
2. To understand methods of diagnosing IFALD, in particular the role of liver biopsy
3. To review management strategies for IFALD, including when to refer for intestinal transplantation
* Disclaimer: BAPEN Position Statements/Guidelines have been prepared as guidance only to assist qualified healthcare
professionals in the decision-making processes surrounding nutritional care. Users of these materials may only do so on the
condition that they exercise their own professional knowledge and skills when applying such guidance to specific
circumstances. Anyone without the appropriate qualifications must seek the advice of a qualified healthcare professional before
taking, or refraining from, any action on the basis of the policies or guidance. BAPEN does not (i) owe a duty of care to users of
the policies or guidance who are not qualified healthcare professionals; and (ii) cannot accept liability to anyone using these
policies or guidance.
Background
Intestinal Failure Associated Liver Disease (IFALD) is a major consequence of long term parenteral
nutrition and chronic intestinal failure (CIF). It was first described in 1971
1
and was previously called
Parenteral Nutrition Associated Liver Disease (PNALD) but was subsequently renamed when it became
understood there were contributions from more than just the PN.
The initial pathology can be predominantly cholestasis, steatosis/steatohepatitis, or a mixed picture
2
,
ductopenia has also been described
3
. Regardless of the initial pathology, progressive fibrosis leading to
cirrhosis can also occur and therefore one of the goals of intensive intestinal rehabilitation is to prevent
progression of IFALD so all members of the multiprofessional Intestinal Rehabilitation teams should be
aware of diagnosis and management of this condition.
Whilst there is no standard definition of IFALD, a summary of previously published diagnostic criteria used
in clinical studies
4–8
are given in table 1.
Who is at risk of IFALD?
Any patient with chronic intestinal failure receiving parenteral support is considered at risk of developing
IFALD. The development of disease is likely multifactorial
9
. PN related factors include amount of energy,
glucose, lipid (including type) delivered parenterally vs enterally, and delivery rate (and whether this
exceeds the glucose oxidation rate). Certain nutrient excesses (including phytosterols, copper,
manganese and aluminium) or deficiencies (choline, taurine, carnitine and essential fatty acids) have been
linked to the pathogenesis of IFALD
10
. Non-PN factors include the frequency of catheter related
bloodstream infections (CRBSI) episodes, the residual gut length (though this may just be a surrogate for

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
the proportion of energy delivered parenterally), the presence of a colon, gallstones,
bacterial overgrowth and drugs
11–17
. In the paediatric population, the predominant
histological finding is of cholestasis, as infants and young children have immature bile
ducts vulnerable to injury. Hence, children can present with jaundice in early IFALD and
this can be reversible with changes to the PN
18
, weaning to enteral nutrition and growth. In
adults, the main histological finding tends to be steatosis with or without steatohepatitis and fibrosis,
though cholestasis often co-exists and ductopenia has been described. Differentiating IFALD from non-
alcoholic fatty liver disease (NAFLD) can be challenging and requires review by an experienced
pathologist in the context of the appropriate clinical history
2
.
The significant risk factors for developing IFALD are ultra-short bowel (generally defined as 20cm of
residual small bowel from the duodenojejunal flexure in adults, or 10cm in children), co-existing alcohol
consumption, diabetes or other pathology associated with liver damage
19
. A small proportion of home
parenteral nutrition (HPN) patients will have chronic viral hepatitis, biliary disease (particularly those with
underlying inflammatory bowel disease) or autoimmune/metabolic liver disease. An under-recognised
group is probably those patients with CIF following complications of bariatric surgery. The prevalence of
steatosis, non-alcoholic steatohepatitis and cirrhosis in patients undergoing bariatric surgery is 91%, 37%
and 17% respectively
20
. These patients may subsequently develop a combination of NALFD and IFALD.
Patients with a second insult to the liver, whether it be NAFLD, alcohol or other intrinsic hepatic or biliary
disease, should be considered at higher risk, though there is insufficient data at the moment to quantify
this.
Table 2 provides a summary of the evidence for risk factors for IFALD
How does IFALD progress?
There is very limited data on how quickly IFALD progresses, and which factors affect this process. Serial
biopsies are very rarely performed and there are only a few reports of this. Two very early case reports
(one in a young adult
21
and one in an infant
22
) demonstrated progression from fibrosis to cirrhosis on serial
biospies. In a recent French study
19
three patients underwent more than one biopsy, and all showed
progression of fibrosis stage whilst continuing HPN. One 24-year-old patient had stage 1 fibrosis
(Kleiner/Brunt score) after 9 months of HPN and stage 3 at 54 months; a 62 year old moved from stage 2
at 18 months to stage 3 at 41 months; finally a 36 year old progressed over 30 months from stage 2 to 3.
In a case series of patients undergoing intestinal transplant, 1/3 showed progression of fibrosis stage
between their baseline/assessment biopsy and an intraoperative biopsy at the time of transplant, after a
median of just 191 days
23
on PN.
It is acknowledged that the development of clinical jaundice is a very late sign in IFALD and prognosis
after this is poor. One older small series showed that death occurred within a median of 10.8 months after
the initial bilirubin rise
24
and transplant centres do continue to see patients with jaundice deteriorate very
rapidly. Mortality on the waiting list for combined liver/intestine transplant has always been high, reflecting
the poor physical condition of patients with two organ failures. In the period 1987 to 2005, 29.8% of
candidates listed in the US for a combined liver and intestine transplant died on the waiting list, compared
to 8.8% of those awaiting intestine only
25
. The most recent report from the US
26
shows the absolute
numbers are better, but the disparity is just as stark, with a mortality of 2.72% of those awaiting an isolated
intestine graft versus 10.3% of those awaiting combined grafts. Wait times are longer for combined liver/
intestine grafts – median 190 days in the UK versus 139 days for isolated small bowel graft
27
.
How is IFALD diagnosed?
Abnormalities in liver biochemistry are commonly seen in the early phase of PN and usually reflect pre-
existing liver disease, drugs or sepsis, rather than an early form of IFALD
9
. True IFALD takes years to
develop, though this can be a short number of years in those at very high risk.
Clinical manifestations of decompensated liver disease in non-IF patients include splenomegaly, ascites
and varices. These signs develop as a consequence of portal hypertension. However, patients with CIF,
especially short bowel, do not exhibit these features even with very advanced liver disease, due to the

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
general reduction in splanchnic blood flow
28
. The development of jaundice in an adult
with IFALD is an end-stage sign and patients often die within months of this
25
.
Intestinal Rehabilitation teams often see abnormalities in liver biochemistry in patients
receiving PN. In the acute hospital setting it is mainly due to drugs, sepsis, or pre-existing
liver disease
9
. In a patient with CIF receiving HPN, a raised alkaline phosphatase is the most common
abnormality
14
, which may be related to metabolic bone disease in many cases, rather than liver disease.
This same study showed a high proportion of patients had abnormal liver biochemistry but none
developed overt liver disease by the end of the study (median follow up 18.5 months). In a study by a
group in France, there was no correlation between moderate or advanced liver fibrosis and the liver
function tests
19
.
Non-invasive tests have gained popularity in other aetiologies of liver disease/cirrhosis, but none have
been validated in IFALD. Transient Elastography (Fibroscan) in particular seems an attractive method,
but 2 studies have shown it is not reliable
29,30
. As the elastography component on Fibroscan relies on liver
stiffness, which will be influenced by portal inflow, it is perhaps not surprising that it is unreliable in patients
with CIF, who do not have normal mesenteric/portal haemodynamics.
Certain biochemical panels have been proposed as non-invasive methods to detect fibrosis, including
Fibrosis-4 (FIB-4), Enhanced Liver Profile (ELF, which measures serum levels of 3 fibrosis markers) and
APRI. The FIB-4 test uses a formula derived from patient age, alanine transaminase, aspartate
transaminase and platelet count, and has been particularly validated in NAFLD
31
, but can be used in other
liver diseases too. In a 2020 IF registry based review, FIB-4 was associated with risk factors for IFALD,
such as bowel length and time on HPN but no comparison with histology or other modalities were carried
out
32
.
The Enhanced Liver Fibrosis (ELF) score likewise can be used in many chronic liver diseases
33
, and is
now recommended in the NICE diagnostic criteria for NAFLD and combines a score from serum levels of
3 fibrosis markers (Tissue Inhibitor of metalloproteinases-1, amino terminal propeptide of type III
procollagen and hyaluronic acid). In a recent study of paediatric patients with CIF
34
, ELF did not correlate
with any known IFALD risk factors (duration of PN, proportion of energy delivered enterally, number of
CRBSI episodes). Adding to this, a study of HPN patients from Southampton
35
showed ELF, FIB-4 and
elastography were unreliable for diagnosing IFALD. There is an opportunity for intestinal rehabilitation
centres to work together in further evaluation of these potential diagnostic tools.
AST Platelet Ratio Index (APRI) is particularly used in chronic hepatitis C and can be easily calculated
from these two blood parameters. It did correlate with some IFALD risk factors in the previously mentioned
2021 study
34
, but there was no ‘gold standard’ comparison. The only other method to show correlation
with IFALD risk factors is the LiMAX test
36
, a dynamic test that measures metabolism of
13
C labelled
methacetin, but this measures liver functional capacity rather than the degree of fibrosis. However, it is
useful in predicting outcome after liver resection and may be worth further evaluation.
Current best practice for diagnosing IFALD, certainly among transplant centres, involves a liver biopsy. A
review of the technique, criteria for establishing adequacy of histology samples and for reporting provides
useful practical tips
37
. There is limited published information available on how well a biopsy represents the
entire liver in IFALD and the histology must always be correlated with the clinical scenario. IFALD can be
difficult to distinguish from NAFLD, though there a few key histopathological differences (Table 3).
Sequential liver biopsies whilst on HPN would be the most informative in determining the trajectory of liver
disease, but are unlikely to be possible or palatable for all HPN patients.
The additional information provided by hepatic venous wedge pressures may justify undertaking this
procedure in selected patients, particularly for those whose liver fibrosis appears to be borderline for
recovery after isolated intestinal transplant. In the UK, current practice in CIF and transplant centres would
place this ‘point of irreversibility’ at severe fibrosis (Ishak 4 or 5
38
). Some previous attempts to perform
isolated intestinal grafts in patients with well compensated cirrhosis and/or absence of portal hypertension
have resulted in acute liver failure and/or death
39
, however, there are also case reports of good long term
outcomes in this scenario
40
.

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
What is the role of Transplant in IFALD?
The only treatment for established cirrhosis due to IFALD is a combined liver and intestine
transplant. Historically, patients were only referred for consideration of transplant when they
had developed overt cirrhosis. Unsurprisingly the mortality for this group of sick patients with advanced
organ failure (both pre and post-transplant), was high. It has become apparent that for patients with pre-
cirrhotic IFALD-related fibrosis an isolated intestinal transplant can halt progression or even reverse the
degree of fibrosis
41,42,43.
Isolated intestinal transplants are technically easier, associated with shorter
lengths of stay, fewer complications and greatly improved survival compared to combined liver and
intestine grafts
24,41,42
(Figure 1). If we can diagnose IFALD at these earlier stages, we can also optimise
organ utilisation.
Hence, intestinal transplant centres have been trying to promote earlier referrals for IFALD, but the only
way to currently diagnose those with early IFALD is with a liver biopsy, an invasive test with a small but
definite risk of complications. We therefore need to think about which patients are at highest risk for IFALD
and consider when to perform liver biopsies on this group of patients.
Who, When and How to biopsy?
Liver biopsies are usually carried out using ultrasound guidance, via a percutaneous approach with a 16
gauge needle. Two passes are generally adequate for sampling. Most are performed as a day case with
minimal-mild post-procedural pain
37
. The main risk that clinicians and patients are wary of is bleeding. A
UK-wide audit in 2013
45
demonstrated that only 0.4% of patients had clinically significant bleeding
following a liver biopsy (being defined as a drop in Haemoglobin, radiological evidence of bleeding or need
for intervention for bleeding. The mortality rate related to bleeding was 0.11% and the deaths all occurred
in patients having targeted lesion biopsies.
Transjugular biopsies are available in some hospitals and can be considered for patients with
coagulopathy or ascites. However, such patients are unlikely to be undergoing a diagnostic biopsy outside
of a transplant centre. It is critical that biopsies are reported by an experienced pathologist, especially as
no standardised diagnostic criteria are available. The pathologist should initially comment on whether the
sample is adequate for diagnosis. American Association for the Study of Liver Diseases (AASLD)
guidelines state a sample with 12 or more portal tracts present should be sufficient for diagnosis
46
. The
aim of histological examination is to confirm or refute the diagnosis of IFALD, stage IFALD and exclude
co-existent liver pathologies. Descriptive reports as well as quantitative estimates of fibrosis (using Ishak
or Brunt scores
38
,
47
) are helpful.
Considering who to biopsy, clearly not all HPN patients should undergo this procedure. If one of the aims
of diagnosing early is to ensure a timely referral for intestinal transplant, the clinician should first consider
whether the patient would be a candidate. There are no upper age limits for transplant in the UK, but the
presence of major cardiorespiratory or neurological disease with poor prognosis, a history of metastatic
cancer or serious psychological morbidity refractory to treatment should be regarded as contra-indications.
If there is any doubt, Intestinal Rehabilitation teams can discuss with a transplant centre.
Thereafter, the individual patient risk must be considered – those who are ultra-short (residual small bowel
length <20cm) are known to be very high risk
15
and should undergo early and possibly sequential
biopsies. This document proposes the second high risk group are those with a second liver insult, as
mentioned above (excess alcohol consumption, previous bariatric surgery or chronic viral, metabolic,
autoimmune or biliary liver disease). These patients should have a baseline biopsy after a relatively short
period of HPN, with further biopsies planned depending on the findings of the baseline sample and the
ongoing presence of the ‘second hit’.

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
Management of IFALD
Comprehensive hepatoprotective strategies should be part of routine care for all patients
with CIF. Clinicians should consider if their patient would be a candidate for intestinal (or
intestine and liver) transplant, how to modify the HPN to be liver-sparing and which non-
PN risk factors can be modified.
Routine IFALD preventative care should, based on the latest available evidence and expert opinion from
BIFA, include the following:
1. Review of HPN script, with particular attention towards lipid type and amount and number of lipid-
free days. Overall lipid intake should aim to be <1g/kg/day wherever possible mixed lipid
emulsions should be used and lipid-free days maximised. Caution must be taken not to over-
burden the patient with excess glucose energy which promotes de novo lipogenesis in the liver.
2. Review medication and stop or minimise all potentially hepatotoxic medications.
3. Restore intestinal continuity wherever possible. This may be of benefit in terms of normalising
entero-hormonal and bile acid signalling as well as calorie-sparing. Along with this, encourage all
patients to take some oral/enteral nutrition
4. Take all measures to reduce the incidence of CRBSI, including re-training, protective caps, single
lumen catheters and antibiotic locks.
5. Counsel patients on their alcohol intake, including reminding them that it can be well absorbed
and potentially toxic, even in patients with a very short bowel.
6. Consider patients who have undergone previous bariatric surgery to be at increased risk and
establish baseline liver function/histology prior to surgery.
7. Screen at risk patients for co-existent liver disease.
Further guidance on management of IFALD is available at https://www.bapen.org.uk/pdfs/bifa/bifa-top-
tips-series-3.pdf and https://pubmed.ncbi.nlm.nih.gov/30017241/
Figure 2 shows a schematic representation of a proposed management algorithm for patients deemed at
high risk of IFALD.
Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
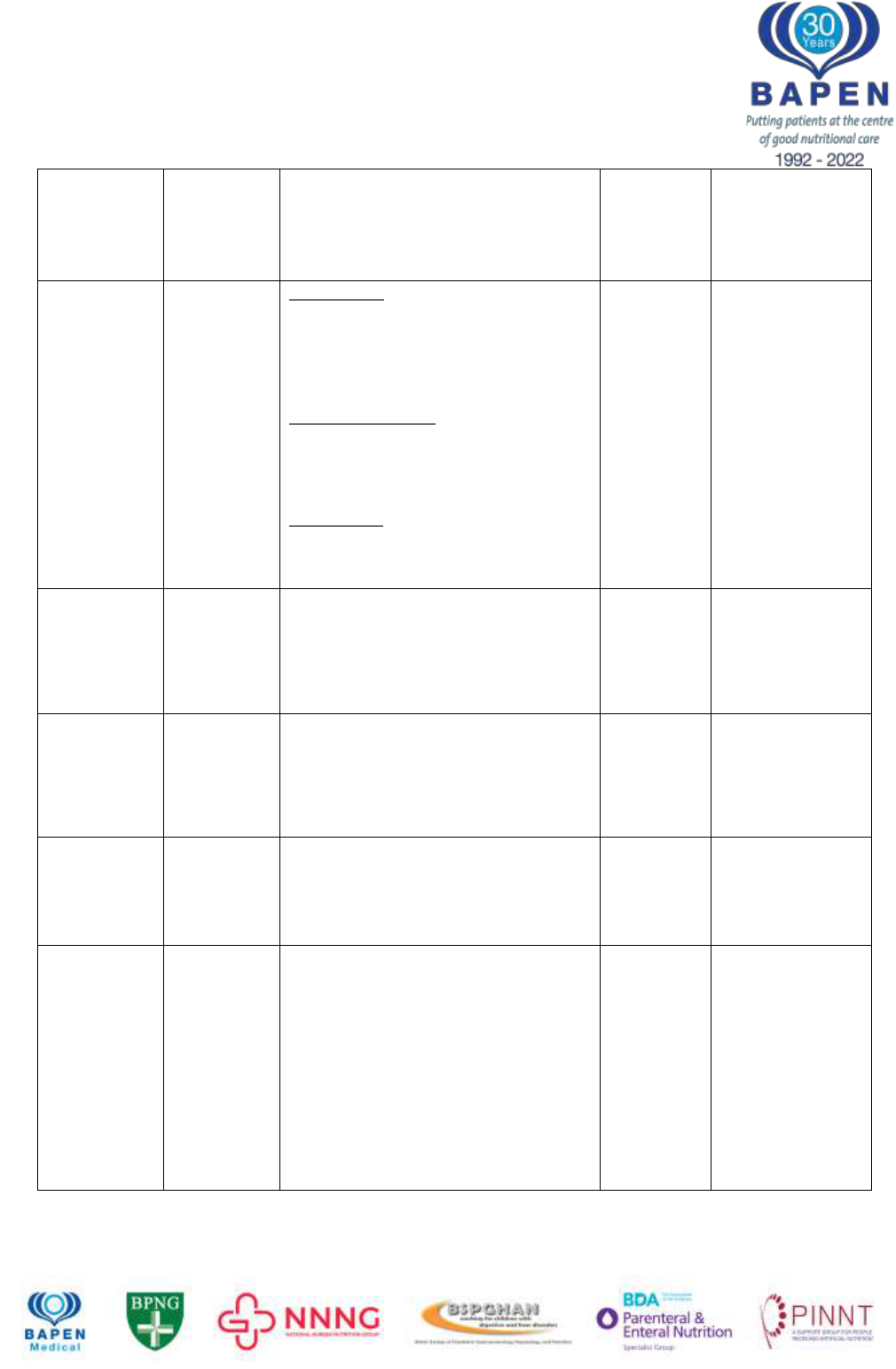
Table 1: Frequency of IFALD according to various studies.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; CBili, conjugated bilirubin; GGT, gamma glutamyltransferase; LBx,
liver biopsy; ULN, upper limit of normal
Citation
Number
and type of
patients
Diagnostic criteria
Population
frequency
according
to these
criteria
Comments
Beath 2008
4
(IRTA
collaboration)
Adults and
children
Early IFALD: ALP and GGT
>1.5xULN for 6 weeks and Bilirubin
<3 mg/dL. If LBx performed, up to
25% of parenchyma will show
steatosis and 50% portal tracts will
show fibrosis
Established IFALD: ALP and GGT >
1.5xULN and Bilirubin 3-6 mg/dL. If
LBx performed significant steatosis
(25%) and up to 50% portal tracts will
show fibrosis
Late IFALD: ALT/AST and ALP >3x
ULN and Bilirubin >6 mg/dL, INR >1.5
and clinical signs of PH. Biopsy
“areas of intense fibrosis”
Abi
Nader
5
2016
N= 251
Paediatrics
ALT or AST or GGT or Bilirubin
>1.5xULN
20%
86 Liver biopsies
in 51 children
Of which 52%
had F2 fibrosis
(18.7% of entire
cohort)
Peyret
6
2011
N=42
Paediatrics
ALT or AST or GGT or Bilirubin
>1.5xULN for 2 months
57%
34 liver biopsies
in 18 children
reviewed:
moderate or
severe fibrosis in
23% of biopsies
ESPEN
guidelines
7
(Lal et al)
2018
All patients
Liver injury as a result of one or more
factors relating to IF including, but not
limited to, PN and occurring in the
absence of another primary
parenchymal liver pathology
Javid
8
2018
N=191
Paediatrics
Conjugated bilirubin >2mg/dL
72%
38% Conj
Bili
>4mg/dL
All-cause
mortality
increased by 3-
fold for baseline
CBili 2-4 mg/dL
(HR 3.25 [1.07-
9.92], p=0.04)
and 4-fold for
baseline CBili >4
mg/dL (HR 4.24
[1.51-11.92],
p=0.006)
Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
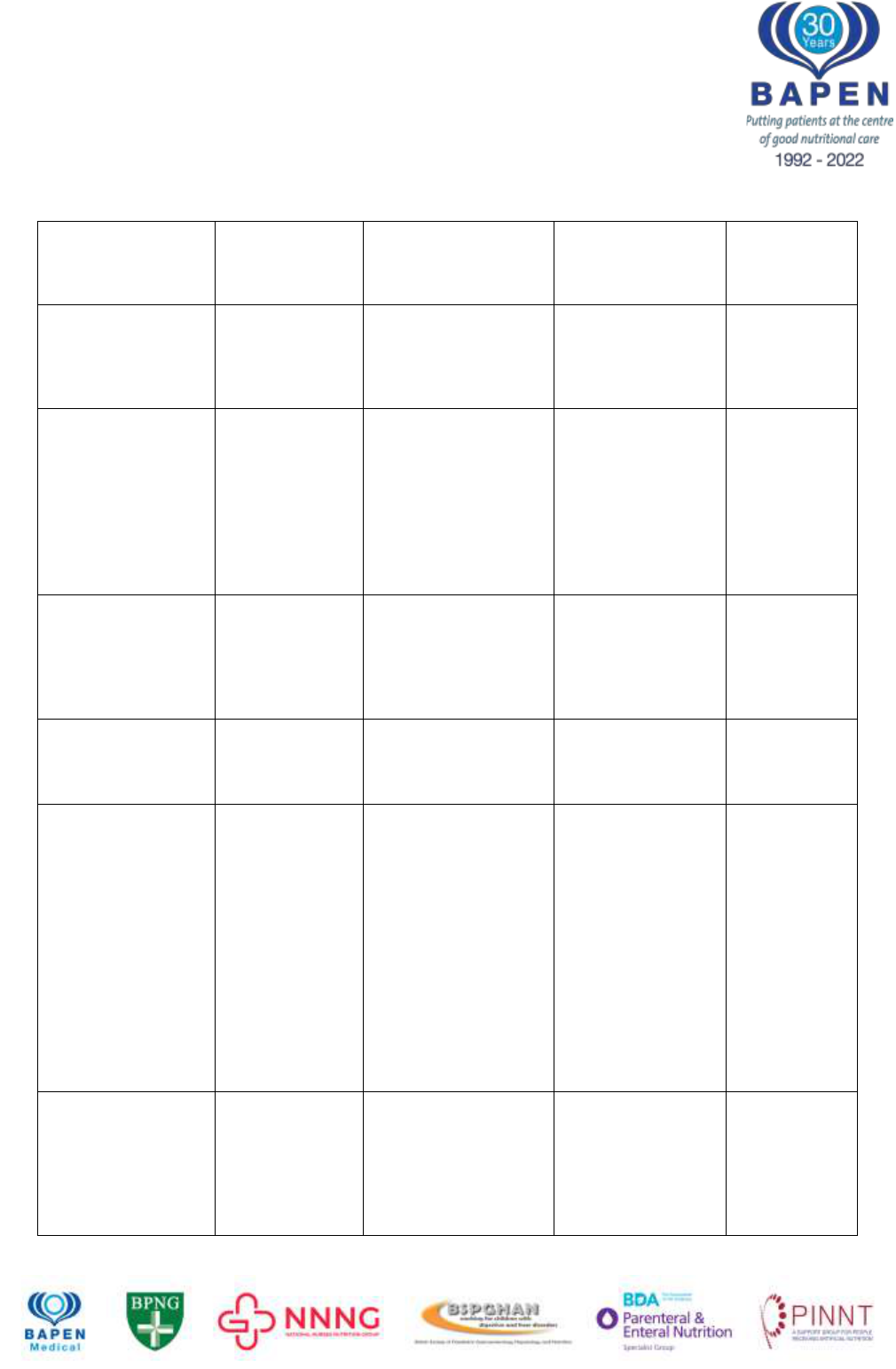
Table 2: Summary of evidence for risk factors in IFALD
CB, Conjugated bilirubin; AST aspartate transaminase; ALT alanine transaminase; TAG,
triacyl glycerol
*Complicated liver disease was defined as extensive portal fibrosis or cirrhosis, jaundice
with a serum bilirubin level of 60mol/L(3.5 mg/dL) or greater for at least 1 month, portal hypertension,
liver encephalopathy, and liver failure
Contributory
factor
Hypothesised
link to IFALD
investigated
Study type / patient
details
Details /
mechanism
Source
Sepsis
General link
between sepsis
and cholestasis
(in all scenarios)
100 adult and
paediatric in-patients
with positive blood
cultures
54% had elevated
serum total
bilirubin levels
Moseley
2004
48
Past incidence
of catheter-
related sepsis is
associated with
severe liver
fibrosis
30 children on long
term PN
Patients stratified by
liver biopsy into
severe fibrosis (group
A) or mild/normal
(Group B)
Incidence of sepsis
was significantly
higher in group A
than in group B
(3.2 +/- 0.3/year vs
1.5 +/- 0.2/year)
Hermans
11
2007
Relationship
between sepsis
and
hyperbilirubine
mia
Post surgical
neonates (n=74)
Episodes of sepsis
associated with
30% increase in
bilirubin
Beath
17
1996
Risk factors for
developing CB
>100mol/L
Prospective study of
152 infants
Odds ratio 3.23 for
septic episodes
(95% CI 1.8-5.9)
Diamond
12
2011
Relationship
between days to
first infection
and cholestasis
/ liver failure
Retrospective study
of 42 inpatients
Mean age at 1
st
infection younger
in those
progressing to liver
failure compared to
those with
cholestasis who
recovered and
those without
cholestasis (28.5
+/- 5 days; 48.2 +/-
14.2; 167 +/- 43.2
days, p<0.01)
Sondheimer
13
1998
Lipid in PN
Absolute
amount of
parenteral lipid
given (soybean)
in relation to
Prospective cohort
study of 90 patients
(adults and children)
Multivariate
analysis:
Parenteral lipid
>1g/kg/day
associated with a
RR of 3.4 for
developing
Cavicchi
15
2000
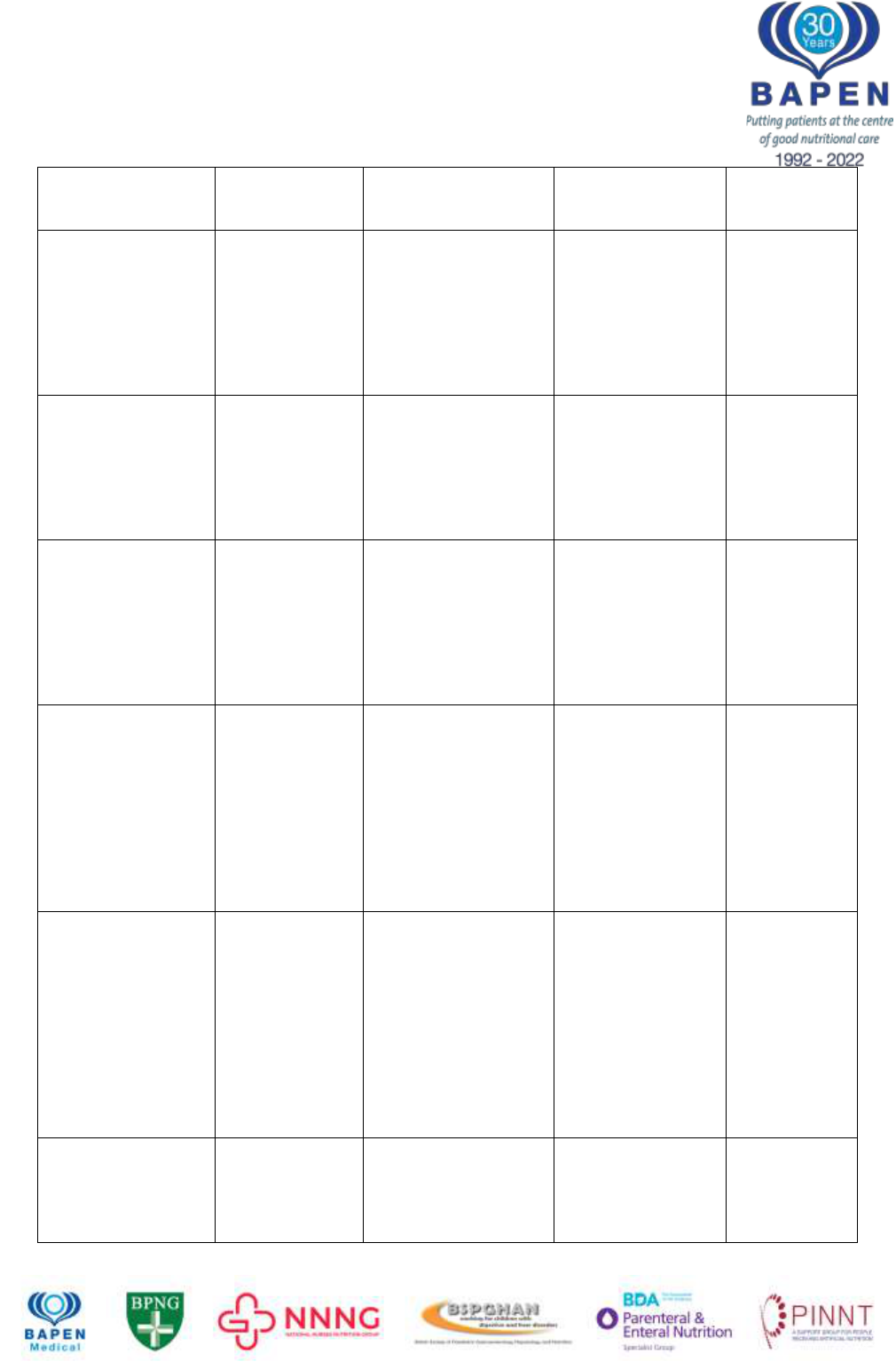
Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
IFALD
development
complicated liver
disease*
Absolute
amount of
parenteral lipid
given (soybean)
in relation to
IFALD
development
Prospective study of
152 infants
10 fold increased
risk of developing
CB >100mol/L
after 60 days if
parenteral lipid
amount >2.5mg/kg
Diamond
12
2011
Reduction of
parenteral lipid
amount
improves IFALD
Prospective study of
1g/kg/day lipid vs
3g/kg/day
Lipid reduction
resulted in
significant decline
in bilirubin
compared to
controls
Cober
49
2012
Reduction of
parenteral lipid
amount
improves IFALD
Retrospective cohort
study of 82 infants
receiving 1g/kg lipid
per day vs 132
infants receiving 2-
3g/kg/day
Multivariate
analysis: standard
lipid amounts 1.77
times more likely to
develop IFALD
than lipid-restricted
infants
Sanchez
50
2013
Type of lipid
influences risk -
Phytosterols
Biochemical analysis
of phytosterol levels
in 29 children
receiving PN (5 with
severe liver disease)
and 29 matched
controls
Very high plasma
levels of
phytosterols in
children with liver
disease compared
to those receiving
PN without liver
disease and
controls
Clayton
51
1993
Type of lipid
influences risk -
Phytosterols
24 patients with short
bowel and 21
controls
Significantly higher
levels of
phytosterols,
cholesterol and
7alpha-hydroxy-4-
cholesten-3-one
Between short
bowel patients and
controls
Ellegard
52
2015
Type of lipid –
fish oil reverses
cholestasis
97 infants diagnosed
with IFALD switched
to fish oil lipid
emulsion
86% survived with
resolution of
cholestasis
Premkumar
53
2014
Date of Preparation August 2022
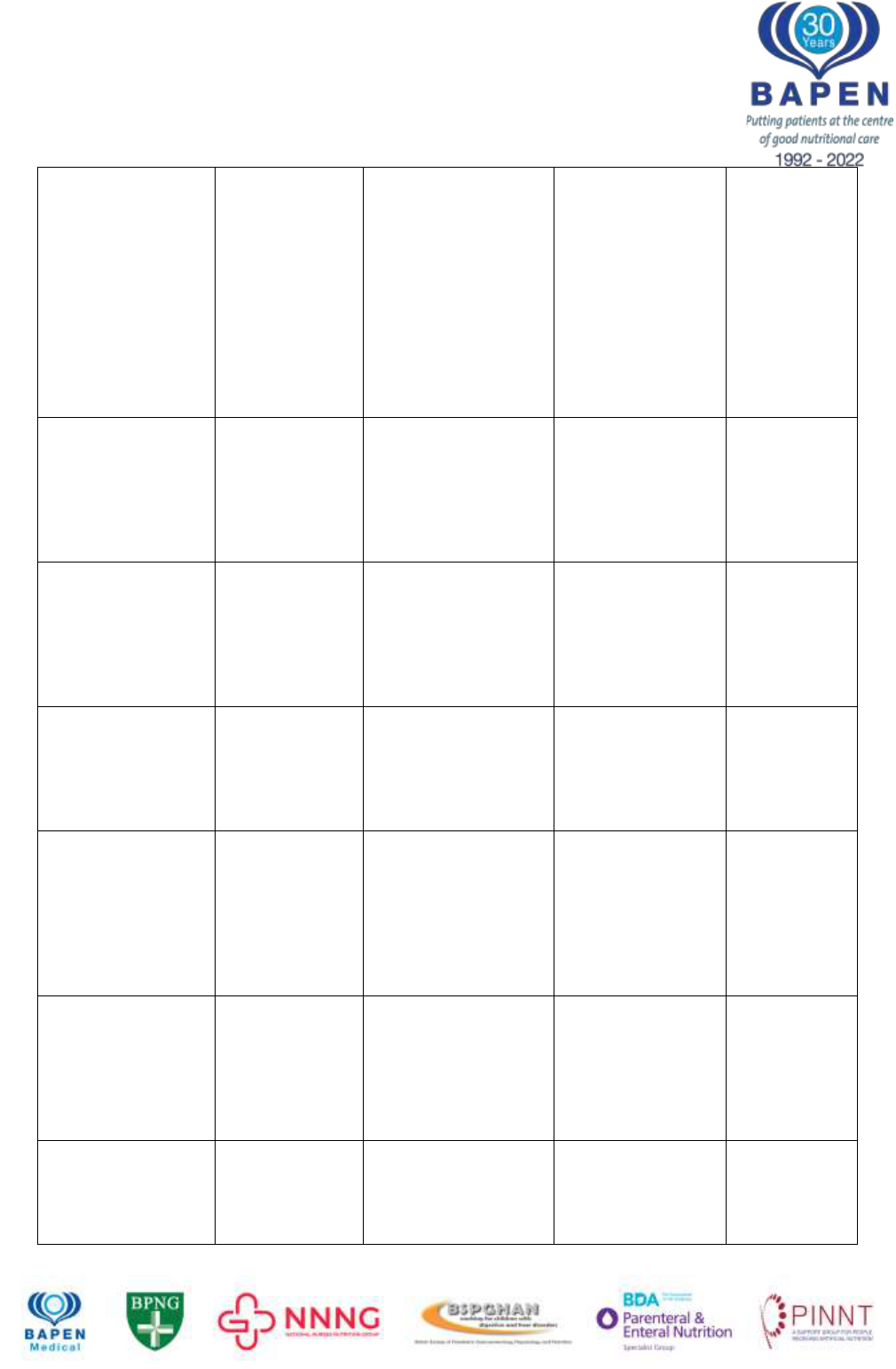
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
Type of lipid –
Mixed lipid
emulsions lower
risk than
Soybean oil
Short term
randomised, double
blind study
34 adult patients
receiving SMOF lipid
vs 39 receiving
intralipid
Significantly lower
ALT, AST and
bilirubin in SMOF
group, significantly
higher -
tocopherol in
SMOF group
Klek
54
2013
Fish oil as a
component of
mixed lipid
emulsion may
be protective
5 year randomised
open label trial of
three intravenous
lipid emulsions
Significantly lower
bilirubin level in
SMOFlipid group
compared to
formulations not
containing fish oil
Klek 2021
55
Parenteral energy
Proportion of
calories
delivered
parenterally
relates to IFALD
risk
113 adults on HPN,
of which 24% had
biochemical chronic
cholestasis (CC)
Higher parenteral
calories intake
associated with CC
(OR 1.2 on
multivariate
analysis)
Lloyd 2008
Influence of
enterally vs
parenterally
delivered
calories
29 patients with IBD
randomised to
isocaloric/isonitrogen
ous PN or EN
Incidence of
deranged LFTs
61.5% in PN group
vs 6.2% in EN
group
Abad-
Lacruz
56
1990
Glucose amount
Fast glucose
infusion
exceeding
Glucose
Oxidation Rate
leads to
steatosis
Patients with burns
injuries
>5mg/kg/minute
glucose infusion
leads to steatosis
Burke
57
1979
Intestinal Anatomy
Residual Small
Bowel length
relates to IFALD
risk
107 adults on HPN
SB length <100cm
significantly
associated with
deranged LFTs on
multivariate
analysis
Luman
14
2002
Residual small
bowel length
relates to IFALD
risk
Prospective cohort
study of 90 patients
(adults and children)
Multivariate
analysis: Small
bowel length
<50cm associated
Cavicchi
15
2000

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
with Relative risk
of 2.1
Presence of
colon protects
against IFALD
development
113 adults on HPN,
of which 24% had
biochemical chronic
cholestasis (CC)
Colon in continuity
protective (Odds
ratio 0.2 on
multivariate
analysis)
Lloyd
16
2008
Nutrient
Deficiencies
Choline
deficiency
impairs hepatic
TAG export,
promoting
steatosis
41 adults and
children receiving PN
Plasma free
choline levels low
in >90% of patients
receiving HPN and
levels correlate
with LFT
abnormalities
Buchman
58
1993
Supplementatio
n of choline
improved liver
disease
15 adult patients
receiving
supplemental choline
Radiological
improvement in
steatosis after
treatment, also
improved ALT,
AST
Buchman
59
2001
Lack of taurine
reduces bile
flow
32 adults receiving
taurine-free HPN with
10 subsequently
receiving taurine-
enriched HPN
Reduction in AST
following
supplementation
Schneider
60
2006
Lack of taurine
reduces bile
flow
Randomised
controlled trial of 236
neonates
Significantly
reduced CB in
certain groups
Spencer
61
2005
Cycling PN
Cyclical vs
continuous PN
associated with
better liver
outcomes
Children with
gastroschisis,
comparison between
continuous PN and
cyclical
Children in
continuous group
2.86 times more
likely to develop
hyperbilirubinaemi
a
Jensen
62
2009
Cyclical vs
continuous PN
associated with
better liver
outcomes
Prospective study of
65 patients with
varying degrees of
cholestasis, half
switched from
continuous to cyclical
PN
Significant
improvement in
biochemical
cholestasis after
switching
Hwang
63
2000

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
Table 3: Distinguishing IFALD from NAFLD (adapted from Buchman et al
2
)
IFALD
NAFLD
Cholestasis
No cholestasis
Macro- and micro-steatosis
Usually macrosteatosis
Zone 1 steatosis
Zone 3 steatosis
Ductopenia and features of biliary
obstruction can be present
Steatohepatitis rare (or mild)
Steatohepatitis common
Fibrosis pattern usually ‘jigsaw’ /
biliary
Sinusoidal fibrosis
Ballooned hepatocytes
Mallory Denk bodies
Figure 1: Kaplan-Meier survival curves (uncensored) for transplantation with and without liver
included (From Woodward et al
64
)
Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
Figure 2: Management scheme for a patient deemed to be ‘high risk’ for IFALD.
‘Standard care’ includes review of PN script, consideration of alternative lipid source,
review of hepatotoxic medications, counselling regarding alcohol intake, attention to
other potentially modifiable hepatic risk factors (see text)
CIF, Chronic Intestinal Failure; USB, Ultra-Short Bowel; ITx, Intestinal Transplant
CIF with USB or 2nd
liver insult
Review of biochemistry
and hepatic imaging to
date
Is patient a candidate
for ITx?
Yes
Perform liver Biopsy
Moderate to severe
fibrosis or cirrhosis
Urgent referral to ITx
centre
Mild to moderate
fibrosis
Discuss with ITx centre,
consider repeat Bx in 2-
3 years
No fibrosis
Continue standard care
with vigilence for
additional liver risk
factors
No
Standard care

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
References
1. Peden VH, Witzleben CL, Skelton MA. Total parenteral nutrition. J Pediatr.
1971;78(1):180-181. doi:10.1016/S0022-3476(71)80289-5
2. Buchman A, Naini B, Spilker B. The Differentiation of Intestinal-Failure-Associated Liver Disease
from Nonalcoholic Fatty Liver and Nonalcoholic Steatohepatitis. Semin Liver Dis. 2017;37(01):033-044.
doi:10.1055/s-0036-1597771
3. Meyerson C, Naini BV. Something old, something new: liver injury associated with total parenteral
nutrition therapy and immune checkpoint inhibitors. Hum Pathol. 2020;96:39-47.
doi:10.1016/j.humpath.2019.10.007
4. Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in
patients with chronic intestinal failure including those who are referred for small bowel transplantation.
Transplantation. 2008;85(10):1378-1384. doi:10.1097/TP.0b013e31816dd513
5. Abi Nader E, Lambe C, Talbotec C, et al. Outcome of home parenteral nutrition in 251 children
over a 14-y period: report of a single center. Am J Clin Nutr. 2016;103(5):1327-1336.
doi:10.3945/ajcn.115.121756
6. Peyret B, Collardeau S, Touzet S, et al. Prevalence of liver complications in children receiving
long-term parenteral nutrition. Eur J Clin Nutr. 2011;65(6):743-749. doi:10.1038/ejcn.2011.26
7. Lal S, Pironi L, Wanten G, et al. Clinical Approach to the Management of Intestinal Failure
Associated Liver Disease (ifald) in Adults: A Position Paper from the Home Artificial Nutrition and Chronic
Intestinal Failure Special Interest Group of Espen. Clin Nutr Edinb Scotl. 2018;37(6 Pt A):1794-1797.
doi:10.1016/j.clnu.2018.07.006
8. Javid PJ, Oron AP, Duggan CP, Squires RH, Horslen SP, Pediatric Intestinal Failure Consortium.
The extent of intestinal failure-associated liver disease in patients referred for intestinal rehabilitation is
associated with increased mortality: an analysis of the Pediatric Intestinal Failure Consortium database. J
Pediatr Surg. 2018;53(7):1399-1402. doi:10.1016/j.jpedsurg.2017.08.049
9. Gabe SM, Culkin A. Abnormal liver function tests in the parenteral nutrition fed patient. Frontline
Gastroenterol. 2010;1(2):98-104. doi:10.1136/fg.2009.000521
10. Nightingale JMD. Hepatobiliary, renal and bone complications of intestinal failure. Best Pract Res
Clin Gastroenterol. 2003;17(6):907-929. doi:10.1016/s1521-6918(03)00108-2
11. Hermans D, Talbotec C, Lacaille F, Goulet O, Ricour C, Colomb V. Early central catheter
infections may contribute to hepatic fibrosis in children receiving long-term parenteral nutrition. J Pediatr
Gastroenterol Nutr. 2007;44(4):459-463. doi:10.1097/MPG.0b013e318031a5c7
12. Diamond IR, de Silva NT, Tomlinson GA, et al. The role of parenteral lipids in the development of
advanced intestinal failure-associated liver disease in infants: a multiple-variable analysis. JPEN J
Parenter Enteral Nutr. 2011;35(5):596-602. doi:10.1177/0148607111413598
13. Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with
intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 1998;27(2):131-137.
doi:10.1097/00005176-199808000-00001
14. Luman W, Shaffer JL. Prevalence, outcome and associated factors of deranged liver function
tests in patients on home parenteral nutrition. Clin Nutr Edinb Scotl. 2002;21(4):337-343.
doi:10.1054/clnu.2002.0554

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
15. Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease
and contributing factors in patients receiving home parenteral nutrition for permanent
intestinal failure. Ann Intern Med. 2000;132(7):525-532. doi:10.7326/0003-4819-132-7-
200004040-00003
16. Lloyd D a. J, Zabron AA, Gabe SM. Chronic biochemical cholestasis in patients receiving home
parenteral nutrition: prevalence and predisposing factors. Aliment Pharmacol Ther. 2008;27(7):552-560.
doi:10.1111/j.1365-2036.2008.03615.x
17. Beath SV, Davies P, Papadopoulou A, et al. Parenteral nutrition-related cholestasis in
postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg. 1996;31(4):604-606.
doi:10.1016/s0022-3468(96)90507-2
18. Puder M, Valim C, Meisel JA, et al. Parenteral fish oil improves outcomes in patients with
parenteral nutrition-associated liver injury. Ann Surg. 2009;250(3):395-402.
doi:10.1097/SLA.0b013e3181b36657
19. Cazals-Hatem D, Billiauws L, Rautou PE, et al. Ultra-short bowel is an independent risk factor for
liver fibrosis in adults with home parenteral nutrition. Liver Int Off J Int Assoc Study Liver. 2018;38(1):174-
182. doi:10.1111/liv.13545
20. Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing
bariatric surgery. J Hepatol. 2006;45(4):600-606. doi:10.1016/j.jhep.2006.06.013
21. Craig RM, Neumann T, Jeejeebhoy KN, Yokoo H. Severe hepatocellular reaction resembling
alcoholic hepatitis with cirrhosis after massive small bowel resection and prolonged total parenteral
nutrition. Gastroenterology. 1980;79(1):131-137.
22. Vileisis RA, Sorensen K, Gonzalez-Crussi F, Hunt CE. Liver malignancy after parenteral nutrition.
J Pediatr. 1982;100(1):88-90. doi:10.1016/s0022-3476(82)80242-4
23. Huard G, Fiel MI, Moon J, Iyer K, Schiano TD. Prevalence, Evolution, and Risk Factors for
Advanced Liver Fibrosis in Adults Undergoing Intestinal Transplantation. JPEN J Parenter Enteral Nutr.
2018;42(7):1195-1202. doi:10.1002/jpen.1148
24. Chan S, McCowen KC, Bistrian BR, et al. Incidence, prognosis, and etiology of end-stage liver
disease in patients receiving home total parenteral nutrition. Surgery. 1999;126(1):28-34.
doi:10.1067/msy.1999.98925
25. Chungfat N, Dixler I, Cohran V, Buchman A, Abecassis M, Fryer J. Impact of parenteral nutrition-
associated liver disease on intestinal transplant waitlist dynamics. J Am Coll Surg. 2007;205(6):755-761.
doi:10.1016/j.jamcollsurg.2007.06.299
26. Smith JM, Weaver T, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Intestine. Am J
Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2020;20 Suppl s1:300-339.
doi:10.1111/ajt.15675
27. NHSBT Organ Specific Reports. https://www.odt.nhs.uk/statistics-and-reports/organ-specific-
reports/
28. Chouhan MD, Lythgoe MF, Mookerjee RP, Taylor SA. Vascular assessment of liver disease-
towards a new frontier in MRI. Br J Radiol. 2016;89(1064):20150675. doi:10.1259/bjr.20150675
29. Van Gossum A, Pironi L, Messing B, et al. Transient Elastography (FibroScan) Is Not Correlated
With Liver Fibrosis but With Cholestasis in Patients With Long-Term Home Parenteral Nutrition. JPEN J
Parenter Enteral Nutr. 2015;39(6):719-724. doi:10.1177/0148607114538057

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
30. Knop V, Neuberger SC, Marienfeld S, et al. Intestinal failure-associated liver
disease in patients with short bowel syndrome: Evaluation by transient elastography.
Nutr Burbank Los Angel Cty Calif. 2019;63-64:134-140. doi:10.1016/j.nut.2019.02.001
31. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of
fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol Off Clin Pract J Am
Gastroenterol Assoc. 2009;7(10):1104-1112. doi:10.1016/j.cgh.2009.05.033
32. Wang J, Wamuo O, Micic D. Evaluation of Fibrosis in Intestinal Failure-Associated Liver Disease
in the Sustain Registry. JPEN J Parenter Enteral Nutr. 2020;44(7):1285-1290. doi:10.1002/jpen.1758
33. Patel PJ, Connoley D, Rhodes F, Srivastava A, Rosenberg W. A review of the clinical utility of the
Enhanced Liver Fibrosis test in multiple aetiologies of chronic liver disease. Ann Clin Biochem.
2020;57(1):36-43. doi:10.1177/0004563219879962
34. Nagelkerke SCJ, Draijer LG, Benninga MA, Koot BGP, Tabbers MM. The prevalence of liver
fibrosis according to non-invasive tools in a pediatric home parenteral nutrition cohort. Clin Nutr.
2021;40(2):460-466. doi:10.1016/j.clnu.2020.05.039
35. Zhao M, Clarke E, Hollingworth T, Patel J, Smith T, Rutter C. Are there new tools to assess
fibrosis in IFALD? Gut. 2021;70(Suppl 1):A1–A262:A1-A262. doi:10.1136/gutjnl-2020-bsgcampus.359
36. Blüthner E, Bednarsch J, Pape UF, et al. Advanced liver function assessment in patients with
intestinal failure on long-term parenteral nutrition. Clin Nutr Edinb Scotl. 2020;39(2):540-547.
doi:10.1016/j.clnu.2019.02.039
37. Boyd A, Cain O, Chauhan A, Webb GJ. Medical liver biopsy: background, indications, procedure
and histopathology. Frontline Gastroenterol. 2020;11(1):40-47. doi:10.1136/flgastro-2018-101139
38. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J
Hepatol. 1995;22(6):696-699. doi:10.1016/0168-8278(95)80226-6
39. Sudan DL, Kaufman SS, Shaw BW, et al. Isolated intestinal transplantation for intestinal failure.
Am J Gastroenterol. 2000;95(6):1506-1515. doi:10.1111/j.1572-0241.2000.02088.x
40. Fiel MI, Wu HS, Iyer K, Rodriguez-Laiz G, Schiano TD. Rapid Reversal of Parenteral-Nutrition-
Associated Cirrhosis Following Isolated Intestinal Transplantation. J Gastrointest Surg. 2009;13(9):1717-
1723. doi:10.1007/s11605-009-0914-7
41. Fiel MI, Sauter B, Wu HS, et al. Regression of hepatic fibrosis after intestinal transplantation in
total parenteral nutrition liver disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol
Assoc. 2008;6(8):926-933. doi:10.1016/j.cgh.2008.04.011
42. McCulloch A, Davies S, Green J, et al. Early Regression of Intestinal Failure Associated Liver
Disease Following Small Intestinal Transplantation: a single centre experience. Transplantation.
2017;101(6S2):S142. doi:10.1097/01.tp.0000521501.40786.f1
43. Hasegawa T, Sasaki T, Kimura T, et al. Effects of isolated small bowel transplantation on liver
dysfunction caused by intestinal failure and long-term total parenteral nutrition. Pediatr Transplant.
2002;6(3):235-239. doi:10.1034/j.1399-3046.2002.01074.x
44. Abu-Elmagd KM, Costa G, Bond GJ, et al. Five hundred intestinal and multivisceral
transplantations at a single center: major advances with new challenges. Ann Surg. 2009;250(4):567-581.
doi:10.1097/SLA.0b013e3181b67725

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
45. Howlett DC, Drinkwater KJ, Lawrence D, Barter S, Nicholson T. Findings of the
UK national audit evaluating image-guided or image-assisted liver biopsy. Part II. Minor
and major complications and procedure-related mortality. Radiology. 2013;266(1):226-
235. doi:10.1148/radiol.12120224
46. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD, American Association for the
Study of Liver Diseases. Liver biopsy. Hepatol Baltim Md. 2009;49(3):1017-1044. doi:10.1002/hep.22742
47. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic
steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol.
1999;94(9):2467-2474. doi:10.1111/j.1572-0241.1999.01377.x
48. Moseley RH. Sepsis and cholestasis. Clin Liver Dis. 2004;8(1):83-94. doi:10.1016/S1089-
3261(03)00134-X
49. Cober MP, Killu G, Brattain A, Welch KB, Kunisaki SM, Teitelbaum DH. Intravenous fat emulsions
reduction for patients with parenteral nutrition-associated liver disease. J Pediatr. 2012;160(3):421-427.
doi:10.1016/j.jpeds.2011.08.047
50. Sanchez SE, Braun LP, Mercer LD, Sherrill M, Stevens J, Javid PJ. The effect of lipid restriction
on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J Pediatr Surg.
2013;48(3):573-578. doi:10.1016/j.jpedsurg.2012.08.016
51. Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, Milla PJ. Phytosterolemia in children
with parenteral nutrition-associated cholestatic liver disease. Gastroenterology. 1993;105(6):1806-1813.
doi:10.1016/0016-5085(93)91079-w
52. Ellegård L, Sunesson A, Bosaeus I. High serum phytosterol levels in short bowel patients on
parenteral nutrition support. Clin Nutr Edinb Scotl. 2005;24(3):415-420. doi:10.1016/j.clnu.2005.01.001
53. Premkumar MH, Carter BA, Hawthorne KM, King K, Abrams SA. Fish oil-based lipid emulsions in
the treatment of parenteral nutrition-associated liver disease: an ongoing positive experience. Adv Nutr
Bethesda Md. 2014;5(1):65-70. doi:10.3945/an.113.004671
54. Klek S, Chambrier C, Singer P, et al. Four-week parenteral nutrition using a third generation lipid
emulsion (SMOFlipid)--a double-blind, randomised, multicentre study in adults. Clin Nutr Edinb Scotl.
2013;32(2):224-231. doi:10.1016/j.clnu.2012.06.011
55. Klek S, Szczepanek K, Scislo L, et al. Intravenous lipid emulsions and liver function in adult
chronic intestinal failure patients: Results after 5 y of home parenteral nutrition. Nutr Burbank Los Angel
Cty Calif. 2021;82:111029. doi:10.1016/j.nut.2020.111029
56. Abad-Lacruz A, González-Huix F, Esteve M, et al. Liver function tests abnormalities in patients
with inflammatory bowel disease receiving artificial nutrition: a prospective randomized study of total
enteral nutrition vs total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1990;14(6):618-621.
doi:10.1177/0148607190014006618
57. Burke JF, Wolfe RR, Mullany CJ, Mathews DE, Bier DM. Glucose Requirements Following Burn
Injury: Parameters of Optimal Glucose Infusion and Possible Hepatic and Respiratory Abnormalities
Following Excessive Glucose Intake. Ann Surg. 1979;190(3):274-285. doi:10.1097/00000658-197909000-
00002
58. Buchman AL, Moukarzel A, Jenden DJ, Roch M, Rice K, Ament ME. Low plasma free choline is
prevalent in patients receiving long term parenteral nutrition and is associated with hepatic
aminotransferase abnormalities. Clin Nutr Edinb Scotl. 1993;12(1):33-37. doi:10.1016/0261-
5614(93)90143-r

Date of Preparation August 2022
BAPEN brings together the strengths of its Core Groups to optimise nutritional care
59. Buchman AL, Ament ME, Sohel M, et al. Choline deficiency causes reversible
hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline
requirement: a placebo-controlled trial. JPEN J Parenter Enteral Nutr. 2001;25(5):260-
268. doi:10.1177/0148607101025005260
60. Schneider SM, Joly F, Gehrardt MF, et al. Taurine status and response to intravenous taurine
supplementation in adults with short-bowel syndrome undergoing long-term parenteral nutrition: a pilot
study. Br J Nutr. 2006;96(2):365-370. doi:10.1079/bjn20061826
61. Spencer AU, Yu S, Tracy TF, et al. Parenteral nutrition-associated cholestasis in neonates:
multivariate analysis of the potential protective effect of taurine. JPEN J Parenter Enteral Nutr.
2005;29(5):337-343; discussion 343-344. doi:10.1177/0148607105029005337
62. Jensen AR, Goldin AB, Koopmeiners JS, Stevens J, Waldhausen JHT, Kim SS. The association
of cyclic parenteral nutrition and decreased incidence of cholestatic liver disease in patients with
gastroschisis. J Pediatr Surg. 2009;44(1):183-189. doi:10.1016/j.jpedsurg.2008.10.033
63. Hwang TL, Lue MC, Chen LL. Early use of cyclic TPN prevents further deterioration of liver
functions for the TPN patients with impaired liver function. Hepatogastroenterology. 2000;47(35):1347-
1350.
64. Woodward JM, Massey D, Sharkey L. The Long and Short of IT: intestinal failure-associated liver
disease (IFALD) in adults-recommendations for early diagnosis and intestinal transplantation. Frontline
Gastroenterol. 2020;11(1):34-39. doi:10.1136/flgastro-2018-101069
